Zerdine Ultrasound Phantoms are relied on for performance and QA testing of B-mode ultrasound systems. A selection of models supports your clinical needs and helps you meet requirements.
Ensure your ultrasound systems are accurately imaging complex cases. Consistent testing with your ultrasound devices means greater confidence in your patient assessments. Plus, an enhanced 4-year warranty is one of the longest warranties available for ultrasound QA phantoms.
Maintain Compliance
The Zerdine Ultrasound Phantoms are generally compatible with AIUM, ACR, AAPM, IEC 62736, IPEM 102, and EFSUMB TQA QA Guidelines.
Suite of Offerings
Our Zerdine Phantoms suite offers precisely spaced pin targets and fibers, and varying surfaces, for testing various applications.

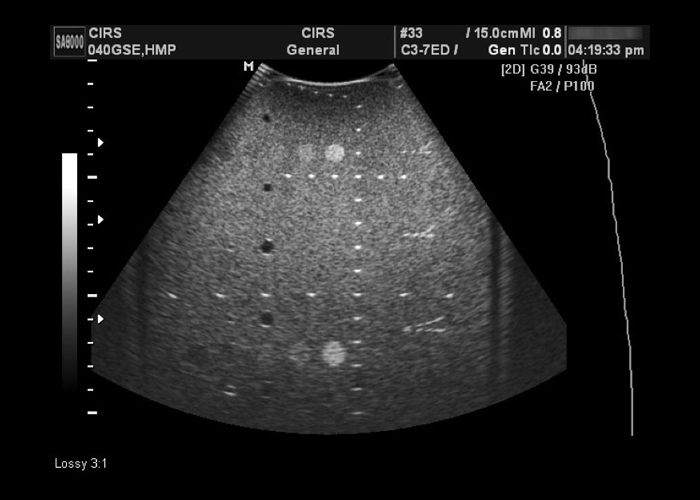
Multi-Purpose, Multi-Tissue Ultrasound Phantom
- Dual attenuation design provides challenging testing environment for high-sensitivity probes
- Detachable water wells allow for testing curvilinear and endocavity probes
- Only general-purpose QA phantom on the market with elasticity targets

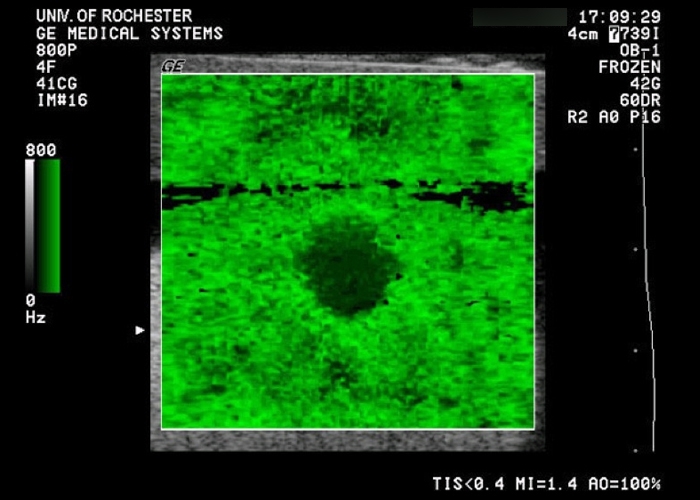
Elasticity QA Phantom
- Four types of lesions with discrete elastic moduli
- Compatible with both shear wave and compression elastography
- For sonoelastography quality assurance

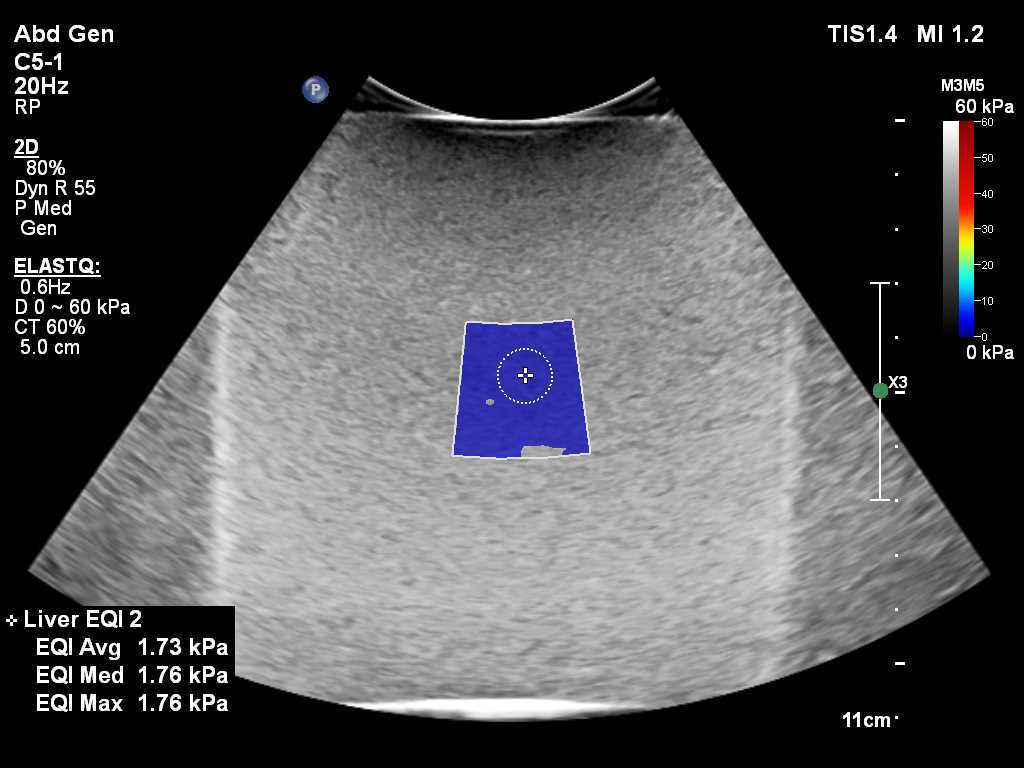
Shear Wave Liver Fibrosis Phantom
- Measure known tissue elasticity
- Set of 4 phantoms, each with a different stiffness
- Enables quantitative assessment of shear wave speed measurements used in the diagnosis of diffuse liver disease
- Designed to comply with QIBA Standards

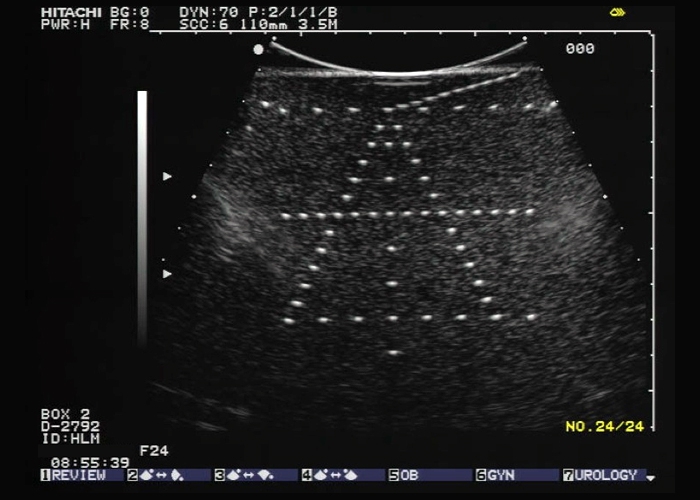
Ultrasound Phantom for 2D & 3D Evaluation
- Perform spatial measurement system checks according to published AIUM standard
- Tests are performed with the aid of wire targets
- Perform Image uniformity and depth of penetration tests.

Accreditation Phantom for Uniformity
- Identify the presence of lateral and/or axial streaks, i.e. artifacts, on any ultrasound transducer
- Simple, compact design makes phantom easy to transport and store
- Soft phantom material conforms to shape of most ultrasound transducers
Specifications
Attenuation Coefficient |
Low: 0.7 dB/cm/mHz; High: 0.95 dB/cm/mHz |
Freezing Point |
0° C |
Melting Point |
Above 100° C |
Speed of Sound |
1540 m/s |
Other |
Compatible with harmonic imaging |
Dimensions |
17.8 cm x 12.7 cm x 20.3 cm (7" x 5" x 8”) |
Weight |
2.8 kg (6 lbs. 5 oz) |
Housing Materials |
ABS Plastic |
Membrane |
Saran-based laminate |
See datasheet for full specifications |
Dimensions |
20 cm x 15 cm x 10 cm (8.5 in x 6 in x 4 in) |
Weight |
6.5 lbs. (3 kg) |
Housing Material |
ABS Plastic |
Membrane |
Saran laminate |
Scannable Surface Area |
17 x 10 cm |
Youngs Modulus |
Background: 18 kPa |
| MoreLess | |
External Dimensions |
Ø 11.6 cm, height 14 cm |
Internal Dimensions |
Ø 10 cm, height 12 cm |
Weight |
6.7 lbs (3 kg) |
Housing Material |
ABS Plastic |
Membrane |
Saran-based laminate |
Scanning Well |
16.5 cm x 10 cm x 1 cm deep |
Density |
1.03 g/cc |
Poisson’s Ratio |
0.5 |
| MoreLess | |
Dimensions |
13 x 18 x 11 cm |
Material |
ABS Housing |
Scanning Membrane |
Saran-based laminate |
Wire Targets |
Material: 0.1 mm Nylon Monofilament |
Dimensions |
Ø 11.2 cm x 7.5 cm |
Weight |
2.2 lbs. (1 kg) |
Materials |
Z-SkinTM elastomer |